RRMS-HDA (High Disease Activity)
Six European clinical neurologists met in December 2015 to finish and validate a model initially constructed by Merck KGaA staff of the benefit-safety balance of six RRMS drugs. After completing the RRMS model, the clinicians replaced some of the original data to reflect research outcomes for patients experiencing High Disease Activity, with results reported here. Click here to open the original RRMS model in a new window for comparing the two sets of results.
The options
The total benefit-safety balance is shown here on a 100-point scale. figures that represent the benefit-safety balance of each. Green best; Red worst.
The total benefit-safety balance is shown here on a 100-point scale. figures that represent the benefit-safety balance of each. Green best; Red worst.
- Cladribine 76
- Alemtuzumab 62
- Natalizumab 61
- Dimethyl fumarate 60
- Fingolimod 49
- Teriflunomide 47
The numbers represent the overall added clinical value of each drug. They take into account available data for the effects and judgements by the experts about the relative clinical relevance of the effects. Reductions in the frequency of HDA symptoms define each drug's favourable effects, and frequencies of side effects are classed as unfavourable effects. The weighted sum of the two figures for each drug gives its overall benefit-safety balance.
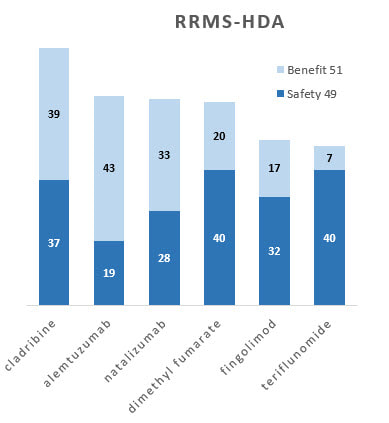
Bar graphs
Separate contributions of benefits and safety to the overall totals show different profiles. For example, after the 51-49 weight is applied show the separate contribution of benefits and safety to the total. Note, for example, that cladribine is best for both benefits and safety, but together they combine to move the benefit-safety balance well above the rest.
Separate contributions of benefits and safety to the overall totals show different profiles. For example, after the 51-49 weight is applied show the separate contribution of benefits and safety to the total. Note, for example, that cladribine is best for both benefits and safety, but together they combine to move the benefit-safety balance well above the rest.
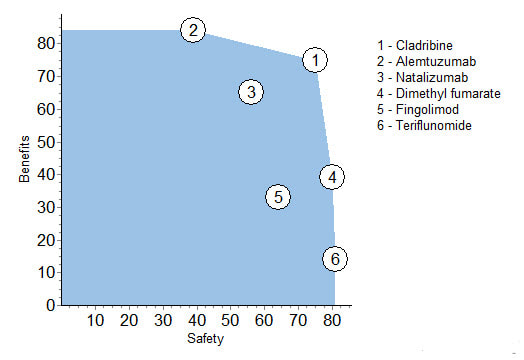
Another way to look at the benefit and safety information is to show each drug's two figures before the benefit-safety trade-off of 51-49 is applied.
The top rating of cladribine is close to being best for both benefits and safety; it is the best drug over a wide range of trade-off weights. Also, cladribine is better in both benefits and safety than 3 and 5, and 4 is better in both than 5.
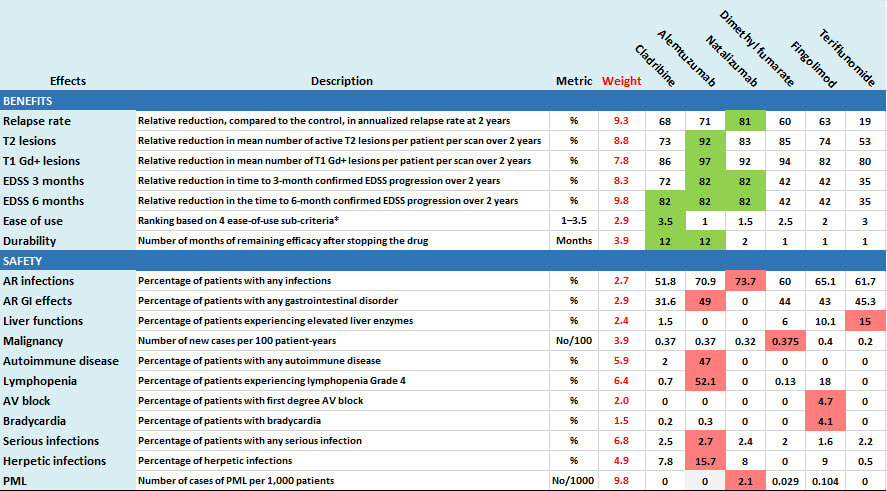
All the above is based on the following data drawn from available public sources, and experts' judgements about the clinical relevance of the data. To support patient-oriented prescribing based on the evidence, green shading identifies the best drug for each favourable effect while red shows the worst drug for each unfavourable effect.
* (1) oral vs iv, (2) few or many doses, (3) monitoring during administration (Y or N) and (4) Co-administration of other drugs (Y or N). Bold Face means preferred.
AR=adverse reaction; AV=atrioventricular; EDSS=expanded disability status scale; Gd+=gadolinium enhanced; PML=progressive multifocal leukoencephalopathy.
AR=adverse reaction; AV=atrioventricular; EDSS=expanded disability status scale; Gd+=gadolinium enhanced; PML=progressive multifocal leukoencephalopathy.
Note that the weighted scores are relative and zero does not mean no value, so only differences between numbers, not their ratios, make sense for comparing the clinical value they represent.
Reference: Vermersch, P., Martinelli, V., Pfleger, C., Rieckmann, P., Alonso-Magdalena, L., Galazka, A., . . . Phillips, L. (2019). Benefit-risk Assessment of Cladribine using Multi-Criteria Decision Analysis (MCDA) for Patients with Relapsing-Remitting Multiple Sclerosis. Clinical Therapeutics, 41(2), 249-260.